Introduction
For over 2000 years, contrast water therapy (CWT) has been a popular tool for treating sports, rheumatic, and orthopedic injuries1,2. This procedure consists of alternating the immersion of a distal segment in warm and cold non-constant water temperatures3-8. Recent studies in athletic recovery9-15 have demonstrated that the use of CWT clearly improves athletic recovery after exhaustive exercise, yet its use in other therapeutic interventions continues to be explored.
In clinical treatment, CWT is conducted in a range between 37°C to 43°C for hot baths and cold baths of 12°C to 15oC which theoretically alternates the area between vasoconstriction and vasodilatation. Versey et al10 (2011) found for athletic recovery that contrast therapy was not dose dependent and improvements were seen after 12 minutes or more of hot and cold CWT. Myrer et al16 (1994) demonstrated that a ratio of 4:1 was sufficient for skin temperature to react in this fluctuation and that the significant fluctuations in subcutaneous temperatures signify the peripheral circulation of the skin caused by contrast therapy7.
This contrasting temperature function in CWT remains unclear in its implication for clinical use. Several studies16-19 have demonstrated that contrast therapy fails to penetrate muscular tissue depths of 1 to 3 cm, therefore not utilizing the physiological and therapeutic effects of heat on tissues6. However, Pournot et al20 (2011) explored the short term physiological effects of different water temperatures after exercise and found that it was the cold water and CWT that were more effective and faster in acute athlete recovery. The therapeutic mechanism suggested by Pournot et al20 is that the cold water portion of the treatment suppressed the inflammatory and damage markers in plasma concentrations reducing the passive leakage by disrupted skeletal muscle resulting in an increase in force production for ensuing exercise. Though in a study by Fiscus21 (2005) the activity of cold therapy had no effect on blood circulation (BC) and that warm water therapy significant increased BC and CWT showed consistent fluctuations in the blood flow during a 20 minute treatment. Unfortunately, therapeutic use of CWT in diabetic patients has been shown in several studies to have little or no vascular response22-24 yet there was an improved response when global heating of the ambient was applied removing the sympathetic vasoconstriction tone of diabetic patient25.
Currently, the interaction between the heating and cooling phases of CWT remains difficult to deduce its therapeutic application in clinical uses outside of athletic recovery. The current protocol in CWT may terminate after either warm or cold application26 depending on the stage of the injury, the desired effect of the treatment, and the ability of the patient to participate. However, the mechanism of alternating between heat and cold at the subcutaneous level remains unclear. In the sub-acute stage, the treatment typically concludes with cold application, whereas treatment of a more chronic condition often concludes with heat application. In short, if vasoconstriction is desired, treatments typically finish with cold; if vasodilatation is desired, treatments end with heat3. Thus the therapeutic process that CWT utilizes may alter the use in clinical treatment, and the sequence in which these therapies should end and begin its application.
In this study, we examined the peripheral circulation of the skin by measuring superficial skin temperatures during and after CWT. We hope to provide insight into the interaction of the hot and cold conditions in CWT in healthy subjects.
Methods
Subjects
The study was submitted to the local ethical committee and was performed after each volunteer gave their informed consent.
Twenty healthy volunteers (12 women/8 men; 21.9±1.7 years old; 62.78±8.4 kg; 1.67±0.1 m, and body mass index 22.7±2.0) participated in our study. Exclusion criteria were: vasomotor instability, loss in superficial skin sensibility, neuropathy, obesity (BMI > 30), systemic and metabolic disease, intolerance to cold or heat, dermatitis and a lower limb injury in the previous six months.
Experimental procedures
Instruments
- Two plastic containers for hot and cold water, containing six liters of water plus 1 kg of ice cubes, and seven liters of warm water with electrical resistance (boiler metal model WE - 1,000 W/127V Resiswal®);
- Digital hygrometer humidity thermometer (Minipa-MT24) to measure the temperature and relative humidity in the room with an accuracy of ± 1°C for temperatures between 0°C and 40°C;
- Thermometer (non-contact infrared thermometer - THERMO TECH®) used to measure skin temperature and water, with an accuracy of ± 2°C.
The 20 volunteers were randomly divided into two groups with 10 volunteers in each protocol (A, B). All volunteers participated as their control condition, with the lower left limb designated for control condition (room temperature), and the lower right limb segment was utilized in the experimental condition (A or B). Skin temperature was measured in the talofibular ligament region always alternating and bilaterally, to ensure that no injury occur.
The volunteers were kept in a sitting position with knees at a 90 degree angle with their feet flat on a dry towel on the floor. The ambient temperature, relative humidity, and the temperature of cold and heated water were measured at the beginning and end of each trial. The average initial temperature of the warm water was 40.4±0.3°C and 10.3±0.2°C for cold water for both protocols (A and B).
Experimental conditions
Both protocol A and B underwent CWT (complete immersion of the right ankle in warm water for 4 minutes followed by immersion in cold water for 1 minute) in 4:1 ratio, 4 sets for a total of 20 minutes. Skin temperature was measured at 5, 10, 15 and 20 minutes. Each trial was taken three times and the average obtained. After 20 minutes of CWT, the volunteers rested in a seated position for 12 minutes in which skin temperature was measured every 3 minutes. All protocols were performed in the same day.
Protocol A (PA) began with warm water and ended the with cold treatment.
Protocol B (PB) began with cold water and ended with the warm treatment.
Study design and statistical analysis
This work was characterized as a quasi-experimental study design with repeated measures. The independent variables were treatment and time, and the dependent variable was skin surface temperature. The repeated measures ANOVA (p<0.05) was considered significant differences in skin surface temperature before and every 5 minutes of application of the protocols A and B, observing the conditions (control / experimental) x time (baseline, 5, 10, 15 and 20 minutes). A post-hoc analysis with Tukey's test (p<0.05) was conducted for each pair of data from both the experimental and control groups.
After the trials, the skin surface temperature continued to be monitored in intervals of three minutes to evaluate the return to homeostasis. This comparison was performed with students t-test p<0.05. All analyzes were performed with the software program Statistica 6.0® and Excel®.
Results
Room temperature and relative humidity
The average temperature in the room was 23.7±1.2°C at baseline and 24.0±1.0°C at the end of trials with no significant difference (t = 0.25, p>0.05). The average relative humidity was 49±5.5% at baseline and 48±6.0% at the end of each trial showing no significant difference (t = 0.45, p>0.05).
Water temperature applied to the volunteers
The initial average temperature of the warm water was 40.4±0.3°C and by the end of the trial dropped to 35.3±1.4°C. For the cold water temperature, baseline was 10.3±0.2°C and by the end of the trial this temperature had increased to 14.2±1.5°C.
Both for warm water and cold water there were significant variations in temperature of the beginning and end of each test (t = 0.000, p>0.05).
Baseline skin temperature
The skin temperature in experimental and control group was measured before application of the contrast bath of which no significant difference on PA (control 31.1±1.7°C and experimental 31.1±1.6°C, p=0.978) and PB (control 31.7±1.9°C and experimental 31.2±1.8°C, p=0.480) was found.
Variation of skin temperature during contrast water bath
The repeated measures ANOVA were applied to determine the overall effect of CWT in the experimental group. There were significant differences in both protocols F (4,72 = 41.860, p=0.000) and F (57,485 = 4.72, p=0.000) for PA and PB was found, respectively. (A and B / control group versus experimental group versus time of application)
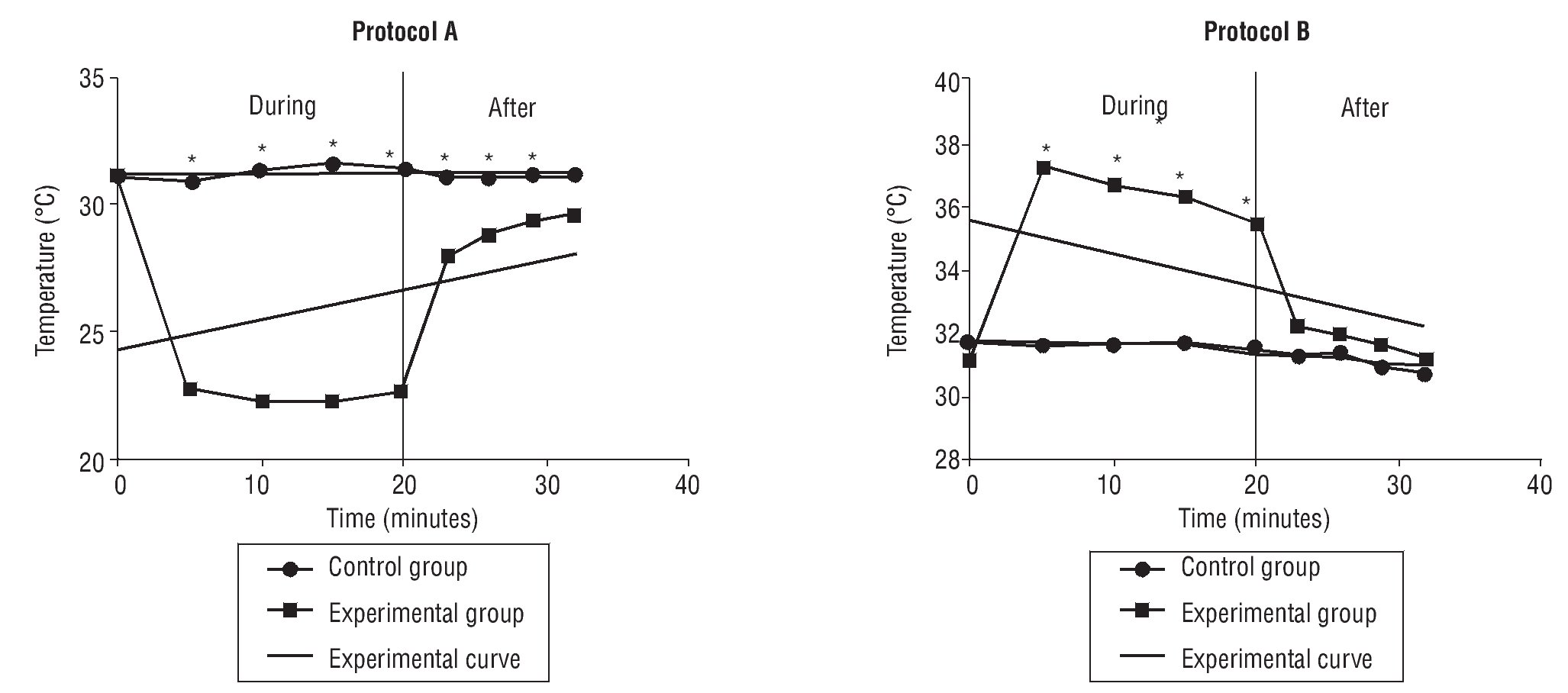
The post-hoc Tukey's test for comparison of means showed significant differences for all pairs of control and experimental groups in PA and PB, at intervals of 5, 10, 15 and 20 minutes of CWT (table 1, fig. 1).
Fig. 1. Variation of skin temperature during and after contrast bath. *Statistical significance p<0.05.
Variation of skin temperature after contrast bath
Analysis of thermal variation of skin after the CWT was performed, in other words, observing the ability of the CWT to maintain the temperature reached.
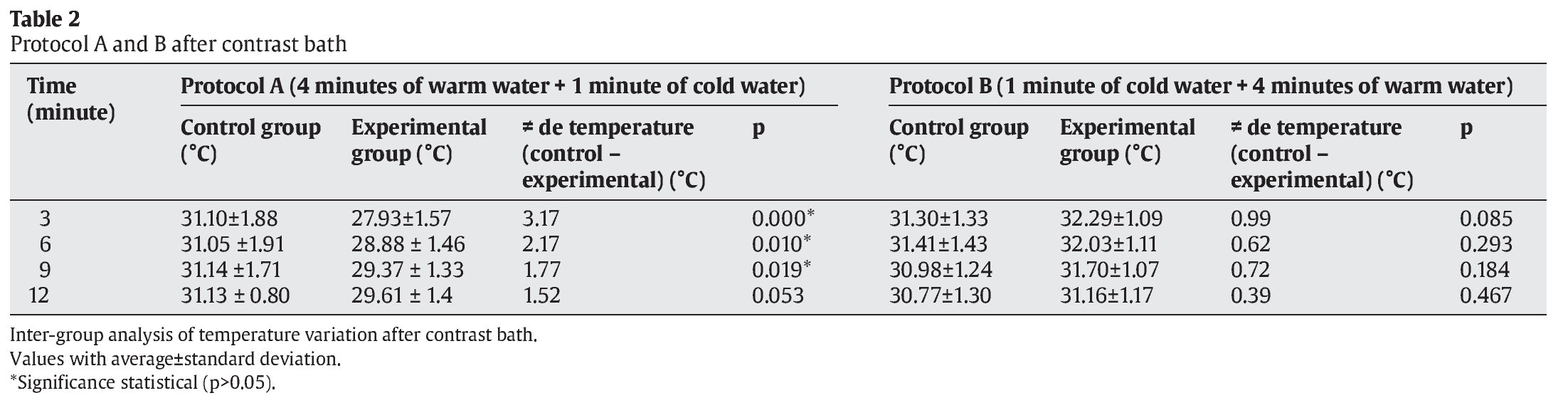
The Student t test was used to compare the average temperatures between the intervals of both control and experimental PA and PB) at 3, 6, 9 and 12 minutes after CWT (table 2, fig. 1).
There were significant differences at 3 minutes (p=0.000), 6 minutes (p=0.010) and 9 minutes (p=0.019) for protocol A, but not at 12 minutes (p=0.053). For PB, no significant differences were found between any conditions or intervals (table 2, fig. 1).
Discussion
CWT has been shown to be an effective tool in athletic recovery after strenuous exercise and its use has been suggested by several textbooks for various other maladies. Several studies have thus suggested that CWT holds benefits such as reduced muscle soreness11, decreased tissue damage9 and increased blood flow21. We suggest that the use of the alternating hot and cold water temperatures provides variability in the use of this treatment which could be more particularly entailed for the ailment.
Water temperature in CWT must be controlled, since it needs to promote significant changes in temperature without causing tissue damage. Current work by Pournot et al20 (2011) illuminates that the mechanism that functions in both cold and CWT involves a suppression in the inflammation and damage markers in the plasma concentrations decreasing plasma leakage of the skeletal muscle. Moreover, Higgins et al9 (2011) whose research implies that damage to the tissue is caused by ice therapy suggests that CWT is an actually more effective treatment for athletic recovery than cryotherapy.
Kotte and Lehmann27 (1994) reported that a tissue must reach a temperature of at least 40°C to produce significant physiological effects. Studies have demonstrated that the temperature changes of tissue during CWT remain at a very superficial level - not reaching 1 cm, therefore maintaining heat on muscle tissue (Van't Hoff's law) is clearly not the therapeutic mechanism being utilized. Fiscus et al21 (2005) identified that heat or warm water therapy as well as CWT has been implicated with increased blood flow, where cold water had no effect on blood flow. However, studies conducted with diabetic patients, whose blood flow was reduced up to 50% to that of their control group, showed that CWT had little to no effect on superficial blood flow. One possible explanation of this comes from Petrovsky et al25 (2006) who found an improved reaction of diabetic patients to CWT when global warming was implemented suggesting that sympathetic vasoconstriction plays a role in their responsiveness to CWT as an effective therapeutic tool. This implies that the heating phase of CWT may contribute to increased blood flow, although not measured in diabetic patients, the improvement to treatment after global warming would suggest that in diabetic patients where the blood flow is reduced a possible longer heating time may be required for the treatment to show effective measures. Our study, which measured the skin temperature after CWT in both protocols at intervals of 3, 6, 9 and 12 minutes supports this idea. PA, managed to maintain the skin surface temperature below homeostasis for up to 9 minutes after treatment had been completed, whereas PB which ended with heated water had returned to homeostasis PB/control group as quickly as 3 minutes post-therapy. A possible explanation for this phenomenon is that the final temperature or exposure time of the heated water or even both were not enough to re-warm the tissues after immersion in cold water. Rodrigues3 (1995) attributes this phenomenon to the thermodynamics of heat exchange in tissues, since the cold tissue takes a "long" time to re-warm. This may explain why a form of global warming where the circulation may already be increased can counteract the effects of the reduced blood circulation we find in some conditions, most notably diabetes. Several studies13,15,28-33 corroborate that the depth of penetration of heat also increases the caliber of blood vessels, in turn blood flow. Thus we suspect that the function of heat in CWT is either a type of protective measure against cryotherapy or increases blood flow or both.
In the present study, ambient temperature and relative humidity were measured and verified to be constant throughout the trials, thus the amount of heat transferred or received by the skin came from CWT, using warm and cold water according to PA and PB. We utilized water with a non constant temperature to replicate clinical practice conditions. The pre and post treatment water temperatures showed a significant change with the hot water dropping close to 5 degrees and the cold water rising approximately 4 degrees. This change in extreme temperatures was clearly seen in our measurements of skin temperature throughout CWT, PB at minute 5, skin temperature reached 37.2°C decreasing to 35.5°C at minute 20. On the other hand, in PA the cold condition maintained efficiency throughout CWT, minute 5 decreasing skin temperature to 22.7°C until minute 20 to 22.6°C. In this study, volunteers reported discomfort with temperatures above 40°C which we considered a factor when deciding our initial temperatures. These results should be considered in clinical practice as one of the key functions of CWT is the alternating temperature extremes which diminishes as CWT is performed.
The present study utilized infrared thermometry as it is a cheap, rapid and non-invasive means of monitoring superficial and core temperature34, with the exception of heat exhaustion35. The choice for use of the infrared thermometry in this research was based on previous studies that showed efficacy in evaluating superficial temperature in cool and hot environments35,36. As well, it has maintained a high correlation with resting mean skin temperatures using contact thermistors (r=0.95)36.
In both protocols (A and B) there were significant variations in skin temperature. The skin, however is an organ that performs heat exchange with ease37 does not imply that this skin variation is heating any of the superficial tissues. Vasomotor stimuli, presumably promoted by CWT, occur in the dermal skin layer, which is highly vascularized16,18,21,38. Fiscus et al21 (2005), suspects that the respective vasodilatation and vasoconstriction of the peripheral blood vessels cause these significant differences between the warm and cold transitions in CWT. However, careful consideration must be made about these results and that these are two distinctly different physiological processes and that further investigation is needed to elucidate their relationship and that by no means measuring one implies that we are measuring the other.
As to whether CWT should end with warm water or cold water we agree with Rodrigues3 (1995), that the choice should be based on the kind and stage of injury, as well as therapeutic targets and metabolic conditions of the tissues. Typically authors16-18,21,29 have conducted research with CWT always beginning with heat and ending with cool devices, as it is the most commonly seen. Our results concur that beginning with warm water and ending with cold water in each set, as shown in PA, is the best form of treatment in cases of sub-acute musculoskeletal injuries - where the objective is to reduce the surface temperature of the skin and reduce the local metabolism. However, in other conditions where there is a compromised blood flow to the lower limb for example, alterations in temperatures, as well as whether the treatment should end with cold or heat should be reexamined for the particulars of the ailment and its effectiveness for the pre-existing conditions. Although limited in its scope, our study would suggest different therapeutic mechanism thus different concerns and considerations should be taken into account with not only injured populations but also those who have conditions affecting blood flow.
Despite our small sample, we consider that our results support other current research being conducted in the area and offers further evidence towards the therapeutic mechanisms of CWT. We suggest that further studies involving CWT to the lower leg in injured populations be carried out to determine whether our findings are clinically relevant and to increase the scientific evidence for the use of CWT, its effects and parameters.
Acknowledgments
To professor Rosa Donadelo for the co-direction throughout the project, and everyone who indirectly contributed to the construction of this research.
Correspondence:
V.W.B. Sá.
Avenida Santa Cruz, 1631 Realengo. Rio de Janeiro. RJ. Brasil.
E-mail:
savagner@ig.com.br
History of the article:
Received May 17, 2011
Accepted August 14, 2011