Objective. The objective of this study was to investigate the effects of 17 weeks of swimming exercise on the lipid profile of hypothyroid rats.
Method. 24 male Wistar rats were divided into four groups: controls submitted to aerobic training (CT); hypothyroid submitted to aerobic training (HT); sedentary controls (SC) and sedentary hypothyroid (SH). HT and SH were induced to hypothyroidism by administering 1 mg of propylthiouracil, while CT and SC animals received distilled water. The animals had unrestricted access to ration and water. Swimming took place five times per week, 60 minutes per session, with overload corresponding to 3% of body weight. At the end of the experiment total cholesterol (C), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), very low-density lipoprotein (VLDL-C), triglycerides and thyroid stimulating hormone (TSH) levels were measured.
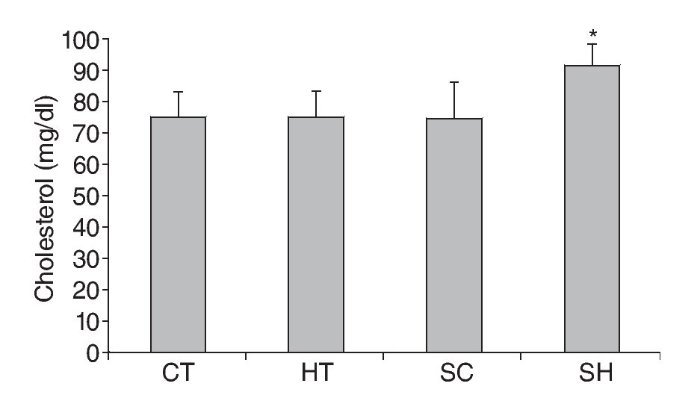
Results. The main finding of the study was the lower values (p < 0.05) obtained for two variables in the HT group (C = 74.6 ± 8.7 mg/dl and LDL = 43.7 ± 6.5 mg/dl) compared to the SH group (C = 91.3 ± 6.8 mg/dl and LDL-C = 55.6 ± 2.0 mg/dl).
Conclusion. It was concluded that swimming exercises can minimize the increase in C and LDL-C blood levels in hypothyroid rats.
Objetivo. El objetivo del estudio fue investigar los efectos de 17 semanas de ejercicios de natación en el perfil lipídico de los ratones de laboratorio con hipotiroidismo.
Método. 24 ratones de laboratorio machos, especie Wistar, fueron divididos en cuatro grupos: control y sometidos a entrenamiento aeróbico (CT); con hipotiroidismo y sometidos a entrenamiento aeróbico (HT); control sedentario (CS) y sedentario con hipotiroidismo (SH). Los animales de los grupos HT y HS fueron inducidos al hipotiroidismo por medio de administración de 1mg de propiltiouracilo, mientras que los animales de los grupos CT y CS, recibieron agua destilada. Los animales tuvieron libre acceso a su alimento y agua. La natación fue realizada 5 veces por semana, 60 minutos por sesión, sobrecarga correspondiente a 3% del peso corporal del animal. Al final del experimento fueron dosificadas las concentraciones de colesterol total (C), lipoproteína de alta densidad (HDL-C), lipoproteína de baja densidad (LDL-C), lipoproteína de muy baja densidad (VLDL-C), triglicéridos y hormona estimulante de la tiroides (THS).
Resultados. El principal hallazgo del estudio fueron los valores menores (p < 0.05) en dos variables del grupo HT (C = 74.6 ± 8.7 mg/dl e LDL = 43.7 ± 6.5 mg/dl) en relación al grupo HS (C = 91.3 ± 6.8 mg/dl e LDLC = 55.6 ± 2.0 mg/dl).
Conclusión. Se concluye que el ejercicio aeróbico de natación puede minimizar en ratones de laboratorio el aumento de los niveles sanguíneos de C y LDL-C debido a la condición de hipotiroidismo.
INTRODUCTION
The thyroid gland is responsible for regulating several metabolic parameters1, such as triiodothyronine (T3) and thyroxine (T4), important hormones for regulating lipid metabolism2,3.
Hypothyroidism is characterized by the decreased release of these hormones, causing secondary dyslipedemia3,4. Patients with this condition exhibit elevated total and LDL-C cholesterol4-7, and increased8,9 or diminished6,10 HDL-C.
Physical exercise has been used as non-drug treatment for the control and treatment of dyslipidemias11. Prado et al.12 report that the use of aerobic exercise, at both low and high intensity, stimulates an increase in lipoprotein lipase (LPL), improving the lipoprotein profile13, and enhancing the enzymatic processes involved in lipid metabolism14. The increase in LPL resulting from aerobic exercise can reduce triglyceride levels and raise HDL-C15. This occurs due to the increased catabolism of triglyceride-rich lipoproteins, leading to a rise in their components and transfer to the high density lipoprotein plasma fraction (HDL-C)16.
On the other hand, the effects of physical exercise on lipid profile in hypothyroidism have not been clarified in the scientific literature. There is evidence that after exercise, circulating TSH levels remain elevated for several days17,18, which could be beneficial in hypothyroidism by increasing thyroid hormone stimulation.
Accordingly, the present study aimed at investigating the effects of a 17-week swimming exercise program on the lipid profile of hypothyroid rats. The hypothesis of this study is that aerobic exercise contributes to minimizing the effects of hypothyroidism on concentrations of total cholesterol and lipoproteins (LDL-C, HDL-C and VLDL-C).
METHOD
The sample was composed of 24 Wistar rats, aged 45 days and weighing between 200 and 250 g, distributed in boxes containing three animals, with free access to water and ration. The environment was monitored under a 12/12 h photoperiod.
This study adhered to the ethical principles of Brazilian Law number 11.794, from October 8, 2008, which establishes procedures for the scientific use of animals. It was approved by the Animal Ethics Committee of Pará State University under protocol number 16/11.
Experimental design
Animals were randomly divided into four groups of six individuals: control and submitted to aerobic training (CT); hypothyroid and submitted to aerobic training (HT); sedentary control (SC) and sedentary hypothyroid (SH).
Propylthiouracil, at a concentration of 1 mg/animal19, was used to induce hypothyroidism. CT and SC group animals received distilled water in the same amount as the propylthiouracil, for the placebo effect, both by gavage administration.
Aerobic training
Training took place in a system of twelve 20-mm tubes placed inside a 500-liter water box, in which the volume of water was planned to prevent animals' tails from reaching the bottom of the box and reducing their effort. Water temperature was maintained between 30 and 32° C to avoid airway problems.
In the week before training, animals underwent a 5-day adaptation period as follows: to the tank, water temperature and handling, in order to minimize stress20.
The training protocol was adapted from a study conducted by Pauli et al.21, consisting of 17 weeks of aerobic training, the first two weeks involving adaptation to the liquid medium. Time progression, initially 15 minutes, occurred in the first week, and overload was introduced in the second week, until reaching 3% of animal body weight. From the third week onwards, animals trained for 60 minutes at 3% overload, five times a week. They were weighed every Monday in order to calculate the weight of the week's aerobic exercise overload. The overload was attached to the chest of the animal with the aid of a vest.
Blood collection
Blood samples were collected to determine lipid profile and TSH concentrations. In the next stage, the animals received no propylthiouracil or distilled water for 48 hours, were denied food for 12 hours, had free access to water and had not engaged in physical exercise for 36 hours.
The animals were then anesthetized with thiopental (40mg.kg1), via intraperitoneal injection. Two mL of blood was collected by suprahepatic inferior vena cava puncture. Lipid profile was determined using the Labtest kit and Trinder's enzymatic technique. The TSH ACS: 180 test with chemiluminescence technology was used to measure TSH.
Statistics
The mean and standard deviation were calculated, as well as measures of data dispersion and variability. The Shapiro-Wilk and Levene tests were employed to check for sample normality and homogeneity. Parametric analysis was conducted for all groups using analysis of variance (ANOVA), while Tukey's post hoc protocol was applied for intergroup comparison. The software used was SPSS version 18, the level of significance used was p < 0.05.
RESULTS
The results of the present study demonstrate that Wistar rats treated with propylthiouracil (HT) submitted to 17 weeks of swimming exercise exhibited lower LDL-C values (p < 0.05) when compared to sedentary hypothyroid (SH) rats. Moreover, the results of trained control rats (CT) and sedentary controls (SC) were also lower (p < 0.05) than those of sedentary hypothyroid (SH) individuals (table 1). The HDL and VLDL-C indices showed no significant intergroup difference after the experimental protocol.
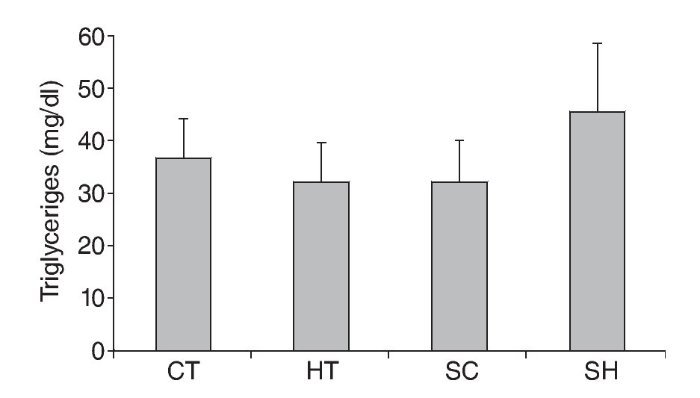
With respect to C, groups CT (75.3 ± 7.7mg/dl), TH (74.6 ± 8.7 mg/dl) and SC (74.3 ± 11.7 mg/dl) displayed lower levels (p < 0.05) when compared to the SH group (91.3 ± 6.8 mg/dl) (fig. 1). Triglyceride values were not significantly different between groups (fig. 2).
Fig. 1. Total cholesterol levels in Wistar rats treated and not treated with propylthiouracil and submitted to 17 weeks of swimming exercises. * p < 0.05 in relation to CT, HT, SC and SC ANOVA, Tukey's Post Hoc. CT: trained control group; HT: trained hypothyroidism group; SC: sedentary control group; SH: sedentary hypothyroidism group.
Fig. 2. Triglyceride levels in Wistar rats treated and not treated with propylthiouracil and submitted to 17 weeks of swimming exercises. CT: trained control group; HT: trained hypothyroidism group; SC: sedentary control group; SH: sedentary hypothyroidism group.
TSH in the HT group (1.7 ± 1.4 mg/dl) showed higher values (p < 0.05) in relation to groups CT (1.1 ± 0.1 mg/dl) and SC (0.8 ± 0.3 mg/dl). Group SH (1.4 ± 0.5 mg/dl) showed a significant difference (p < 0.05) compared to group SC (fig. 3).
Fig. 3. TSH level in Wistar rats treated and not treated with propylthiouracil and submitted to 17 weeks of swimming exercises. * p < 0.05 in relation to CT ANOVA, Tukey's Post Hoc; †p < 0.05 in relation to SC ANOVA, Tukey's Post Hoc; CT: trained control group; HT: trained hypothyroidism group; SC: sedentary control group; SH: sedentary hypothyroidism group
DISCUSSION
Hypothyroidism is one of the primary causes of secondary dyslipidemia3,4, a condition in which lipid metabolism increases due to a rise in total cholesterol and LDL-C4-7, 22-25. However, this increase is not well elucidated in the literature26. The study carried out by Duntas24 demonstrated that other lipid profile alterations in hypothyroidism occur in triglycerides and VLDL-C, whose levels are normal or elevated. The behavior of HDL-C in hypothyroidism is highly variable. While some authors have reported a normal or elevated state8,9,24, others have shown it to be decreased10,27.
In hypothyroidism the number of hepatic receptors that remove LDL-C particles decreases, raising plasma LDL-C cholesterol levels11, possibly accounting for these alterations. There is also an increase in intestinal cholesterol absorption28 and greater hepatic cholesterol synthesis and VLDL-C fraction29.
The results of this study indicate an association between hypothyroidism and lipid alterations. The main finding was the fact that 17 weeks of swimming contributed to controlling lipid profile alterations in hypothyroid-induced rats, such as lower LDL-C and total cholesterol values, when compared to rats with sedentary hypothyroidism. The hypothesis of the present study was confirmed, since aerobic exercise minimized the effects of hypothyroidism, such as increased LDL-C and total cholesterol.
Physical exercise is considered an important non-drug tool for treating dyslipidemias, and aerobic programs (walking, running, swimming) should be used to prevent and rehabilitate patients with dyslipidemia and those recovering from cardiovascular events11. Other authors have also associated regular aerobic exercise to modifications in lipid profile levels12,30,31. On the other hand, studies on the effects of exercise on lipid profile in hypothyroidism remain scarce.
In the present study, 17 weeks of swimming contributed to controlling LDL-C levels in hypothyroid-induced rats when compared to the sedentary hypothyroid group, suggesting that aerobic exercise can improve lipid profile. One of the biomolecular explanations may be the increase in protein lipase activity provoked by exercise13. Seip et al.32 observed a rise in protein lipase gene expression in men submitted to 13 consecutive days of exercises with a subsequent decrease in cholesterol, triglycerides and LDL-C. Moreover, moderate exercise, such as the intensity used in the present study, maximizes fatty acid intake, resulting in lower LDL-C levels in animals that engaged in swimming exercises33.
Reductions in LDL-C levels are important for delaying or inhibiting atherosclerosis, allowing plaque stabilization with a lower risk of erosion, in addition to being essential for significantly improving endothelial function34. However, we suggest future studies to elucidate the possible increase in protein lipase and fatty acid intake chronically induced by exercise in hypothyroidism, given that we were unable to study these mechanisms in the present study.
In regard to total cholesterol, hypothyroid groups exhibited higher values than those of control groups without hypothyroidism. However, chronically, aerobic exercise seems to promote lower cholesterol in hypothyroidism, since the trained hypothyroid group showed decreased total cholesterol values when compared to the sedentary hypothyroid group. The effect of exercise on reducing cholesterol has been widely reported in the literature35-37.However, this chronic training effect in hypothyroidism is still unknown. We suggest future research to confirm the effectiveness of exercise in lowering total cholesterol in hypothyroidism.
Triglyceride values did not differ significantly between groups, but those of the CT, HT and CS groups were very similar. The HS group showed higher triglyceride levels, although the difference was not significant. Hernandez-Mihares et al.38 found increased triglyceride levels in women with hypothyroindism. Similar results were obtained by Kvetny et al.39 and Lai et al.40 but not by Hueston et al.41. Thus, new research should be conducted to confirm the efficacy of physical exercise as a mechanism to control triglyceride levels.
TSH was high in groups with induced hypothyroidism, when compared to control groups. Elevated TSH is a compensatory effect to increase T3 and T4 production, given that hypothyroidism occurs as a function of decreased thyroid hormones. However, propylthiouracil, the drug used to induce hypothyroidism, inhibits type 1 iodothyroninedeiodinase enzyme, which is responsible for converting the T4 hormone into T3, thereby reducing both42,43.
The data in this present study show that aerobic exercise may increase TSH production. The rise in TSH could be associated to greater T3 and T4 degradation in muscle cell membranes, thereby decreasing circulating T3 and T4 and, through negative feedback, elevating TSH production in the hypothalamus and, in turn, raising T3 and T4 production. However, as these hormones were not measured in the present study, we recommend future studies to assess the influence of an exercise-induced increase in TSH on elevated T3 and T4 in hypothyroidism.
Under these study conditions, we conclude that, after 120 days, the use of propylthiouracil at a concentration of 1mg/animal induces hypothyroidism, and that regular swimming exercise can minimize the increase in total blood cholesterol and LDL-C levels. However, there were no hypothyroidism-related changes in HDL-C, VLDL-C or triglyceride concentrations.
ACKNOWLEDGEMENTS
We thank Jusábdon Naves Cansado and Wanderly Fernandes de Miranda, owners of the Labnort Clinical Laboratory, and Centro Universitário UnirG, which supplied the facilities to conduct this study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
History of the article:
Received: September 8, 2012 Accepted: January 29, 2013
Correspondence:
E. Fernandes de Miranda.
E-mail: eduardounirg@gmail.com