Introduction
Performing a motor action like reaching and grasping a cup of coffee on a table usually requires the recruitment of different joints and muscles in a complex pattern due to the many degrees of freedom of our body joints1. The control of these movements implies in recruiting those muscle and joints related to the task in a way that provides a low cost of energy and in an efficient trajectory2. Therefore, by controlling the timing and the intensity of contraction of those muscles involved in the task - for example, by means of muscle activation pattern or muscles synergies - it is possible to obtain adequate patterns of movement3,4.
Handedness is defined as a preference or consistent use of one of hands while performing specific tasks like reaching and grasping5,6. In accordance with Kloppel et al7, some variables present a significant impact on the pattern of motor activation regarding handedness (or dominance), such as the complexity of the task, environmental factors and bimanual actions. Teysedre et al8 suggest that in precision tasks involving the upper limbs, the muscle activation pattern is expressed in a distinct manner between dominant and non-dominant arms, with the last one showing a higher electromyographic (EMG) activity.
Different methodological approaches have been applied to the evaluation of some mechanisms underlying muscle activation pattern related to manual as well as in postural control and other motor tasks9,10. The level of muscular recruitment can be extracted by a very simple, although robust, parameter: the root mean square (RMS) value, which provides information concerning the EMG signal amplitude. Therefore, the aim of this study was to verify the existence of changes in the myoelectric activity of biceps brachii and extensor carpi radialis longus muscles from the dominant and non-dominant arms in a simple manual task, by means of RMS analysis.
Methods
Subjects
Fourteen healthy male subjects (25.2 ± 2.4 years), all right-handed - classified by means of the Edinburgh Handedness Inventory11 - and without neurological and/or muscular - skeletal diseases participated in this study. They are all physical education students but without any specific familiarity in performing the task. The study was submitted to the local ethical committee and was performed after each volunteer gave their informed consent.
Electromyography
The surface electromyographic (sEMG) signal of the extensor carpi radialis longus (ECR) and biceps brachii (BB) muscles was collected through a Miotool 400 acquisition system (Miotec, Ltd; 14 bits; gain: 1,000; active electrodes) by means of surface electrodes (Ag/AgCl, Meditrace 100; 1 cm diameter; interelectrode distance of 30 mm). The sampling frequency was set at 2 kHz. The electrodes were placed over those muscles bellies12-14 following the SENIAM recommendations in agreement with the International Society of Electrophysiology and Kinesiology (ISEK)15. The skin was previously shaved and cleaned with neutral soup. These muscles were chosen based on their relevance in the movements of shoulder, elbow and wrist joints.
Experimental procedures
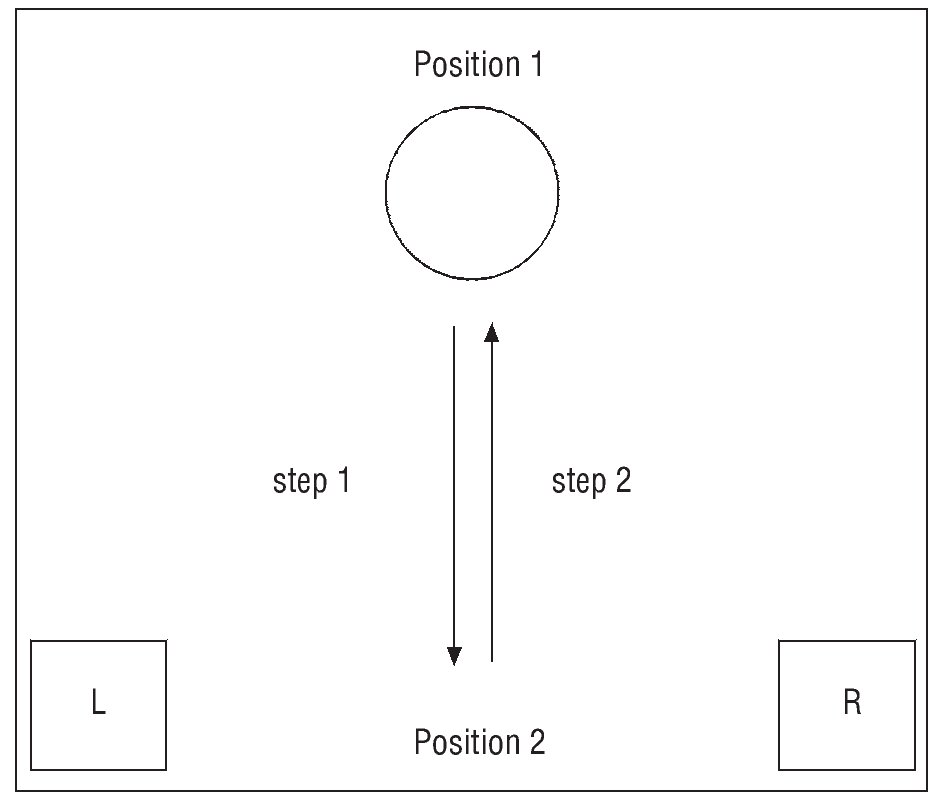
As all the subjects were right-handed (Edinburgh Handedness Inventory12), and thus the right arm was assigned as dominant and the left arm was assigned as non-dominant. The subjects were positioned in a sit position in front of a table (120× 86 cm) adjusted on the height of their chest. The experiment consists of a manual task of moving a cylinder (21 and 25 cm of circumference and length, respectively; 100 g weight). The manual task consists of the following steps: from the initial position (L for the left hand, R for the right) get the cylinder in the position 1 and take to the position 2 (step 1, fig. 1), and return the hand to the initial position; then the subject must get the cylinder in the position 2 and bring back to the position 1 (step 2), and return the hand to the initial position. For sEMG signal analysis the step 1 (bring close to the body) and step 2 (take away) were taken in account. The cylinder positions 1 and 2 were placed 75 cm and 20 cm apart from the subject, respectively. The volunteers were asked to perform the task five times with each arm (randomly assigned), in a comfortable speed. The first and second trials were excluded from the analysis, being considered as a training trial. The forth and the fifth trials were also excluded for analysis to avoid «fatigue». The sEMG signal was collected continuously, being only interrupted when all trials were completed.
Fig. 1. Sequence performed during the manual task. The figure shows an example of a single cycle of the task: get the cylinder in the position 1 and bring to the position 2 (step 1); get the cylinder in the position 2 and bring back to the position 1 (step 2). The task was the same for both arms.
The surface electromyographic data analysis and statistics
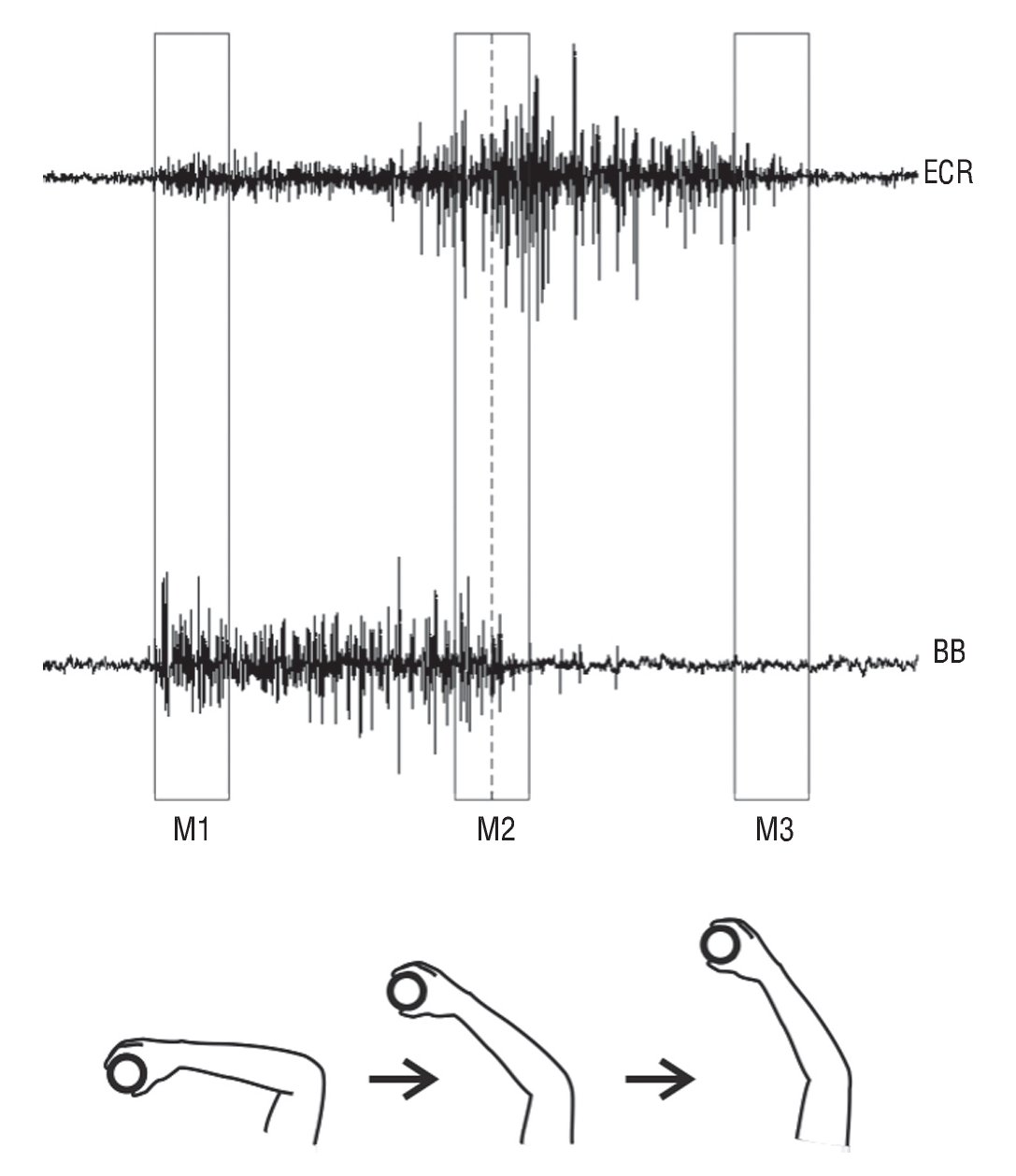
The analysis of sEMG signals data was performed in step 1 and 2 of the manual task. The sEMG signal step decomposition was obtained by visual inspection of the raw data, and using the corresponding time interval for the whole step, which was monitored by one of the experimenters. This decomposition was possible because previously to beginning the task, the volunteers were instructed to take their hands to the positions L and R (fig. 1) and relax. Therefore, the transition between a relaxation and contraction allowed the identification of the transition to the onset of muscle contraction (fig. 2). Further, the beginning (M1) and the end (M3) times were identified (fig. 2). Since there was not a trigger signal to determine M1 and M3 as previously mentioned, they were identified by visually inspecting the raw EMG pattern related to each time during the task. Therefore, both were represented by bursts of raw EMG signal that were closer to each time. As the time spent in the task slightly differ from one subject to another, the median of the sEMG samples of each step was used to determine the intermediate time (M2) of the step. It meant that the data recorded provided a file with a numerical sequence of values (samples) in microvolts, which could be opened and evaluated by software (Excel) that was able to find its median value and that was used as a temporal mark. This file contained data from the beginning of the contraction and the end of that and so these parts were previously cut for the analysis. In the next step, the median was found and M2 (middle or intermediate) was identified as the data window from five samples before and after that value. The beginning (M1) and the end (M3) should be first and the last ten samples, respectively, and so equidistant from M2. It is important to highlight that the three times (M1, M2 and M3) were arbitrarily defined as previously mentioned and presented in figure 2. Besides that, the ten samples of the sEMG signal in each time were extracted to calculate the root mean square (RMS) value (equation 1).
Fig. 2. This schematic view illustrates how the surface electromyographic signal for each time (M1, M2 and M3) was identified during the task, for the step 2 (take away). The bottom of the figure illustrates each time of the task, for the right dominant arm. The rectangular window represents 10 samples of sEMG data, used for the root-mean-square (RMS) value calculation. The dotted line in M2 refers to the median of the sEMG data. BB, biceps brachii; ECR: extensor carpi radialis longus.
Where, N is defined as the number of samples (N = 10); and x, the times (M1, M2 and M3) of the sEMG signal with the samples used for analysis.
To compare the RMS values a repeated measures three-way analysis of variance (ANOVA) was applied, with steps (1 and 2), arms (right, dominant; left, non-dominant) and times (M1, M2 and M3) as within factors. The Tukey-HSD post-hoc test was performed if a significant F value was attained. The level of significance was set at p ≤ 0.05.
Results
The results were presented separately for each muscle (ECR and BB), comparing the two steps (step 1, bring close to the body; step 2, take away), the two arms (right, dominant; and left, non-dominant) and the three times (M1, M2 and M3). The results are also presented in terms of mean and standard deviation (SD).
Extensor carpi radialis longus muscle
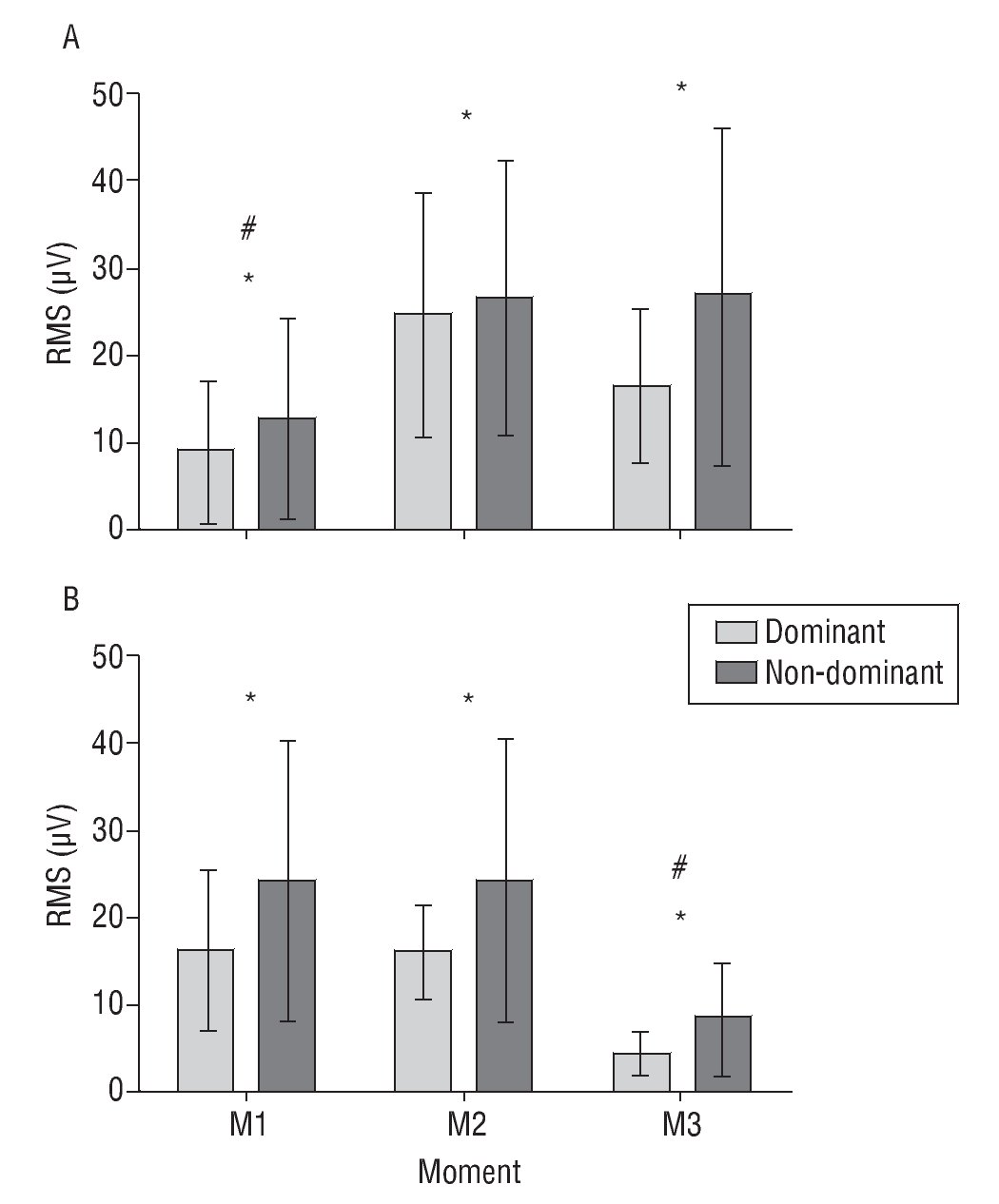
There was a significant effect of step (F(1,3) = 6.49, p = 0.024), arm (F(1,13) = 9.98, p = 0.008) and time (F(2,26) = 30.07, p < 0.001), and a significant interaction step*time (F(2,26) = 16.67, p < 0.001). The RMS value was higher during step 1 (bring close to the body) related with step 2 (take away), and in the non-dominant arm compared with the dominant one (fig. 3). M1 shows the lowest RMS in the step 1, with no difference between M2 and M3. In the other side, RMS in M1 and M2 was higher than M3 during step 2.
Fig. 3. The root-mean-square values (RMS) (mean and standard deviation) from the extensor carpi radialis longus muscles in step 1 (bring to close, A) and step 2 (take away, B), in the dominant and non-dominant hand. *Significant side effect, p < 0.05. #Significant time effect, p < 0.05. The step effect was not shown. See text for further explanation.
Biceps brachii muscle
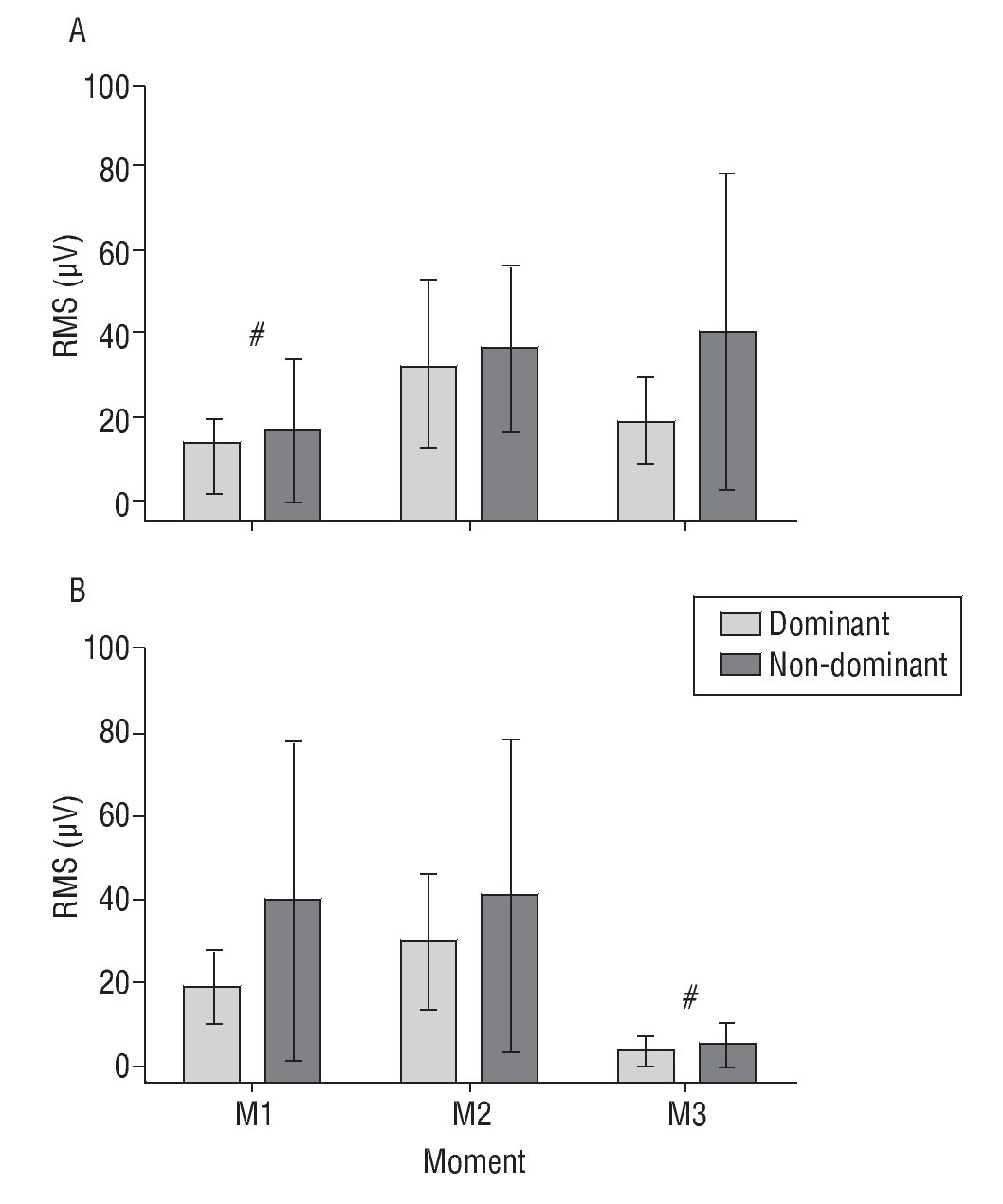
We can observe a significant effect of time (F(2,26) = 56.86, p < 0.001) and an interaction step*time (F(2,26)= 8.79, p = 0.001, see fig. 4). In step 1 (bring close to the body), the RMS shows no differences among M1, M2 and M3. Otherwise, in step 2 (take away), M1 was equal to M2, with M3 being lower than both.
Fig. 4. The root-mean-square values (RMS) (mean and standard deviation) from the biceps brachii muscle in step 1 (bring to close, A) and step 2 (take away, B), in the dominant and non-dominant hand. #Significant time effect, p < 0.05. See text for further explanation.
Discussion
The present study aimed to investigate the level of muscular recruitment in a simple manual task that involves many degrees of freedom. The main results point out that for the ECR muscles, there are significant differences in sEMG from the dominant to non-dominant arm, with an increase in the magnitude and variability of myoelectric signal in the non-dominant side. The difference between dominant and non-dominant arm was not observed in the RMS analysis of BB muscles.
The differences in ECR and BB sEMG activity related to the handedness could be explained by the functional characteristics of these muscles: while ECR is an important muscle in wrist extension and stabilization, the BB has a chief role in elbow flexion. Given this functional role, we could expect that the ECR activity could be a better discriminator of handedness, at least for the manual task used in this experiment. On the other hand, the general role of BB in elbow movement weakens the use of this muscle to distinguish the dominant from non-dominant arm.
The analysis of RMS value in different time during the manual task implies that the temporal pattern of muscle activation are not affected by handedness - at least in this simple manual task - since we did not found any interaction between arm and time. Related to the task itself, it seems that the position in which the arm are extended (M1 in step 1, and M3 in step 2), promotes the lower muscle activation, as pointed by the lower RMS value attained.
Our results corroborate those ones found by Diederichsen et al6, whom investigated movements of flexion and external rotation of the shoulder by means of sEMG signal. They observed a lower myoelectric activity in the shoulder muscles of the dominant side while the volunteers performed flexion movements. However, they found a higher activity during the external rotation of these muscles in the dominant side, which demonstrate that different patterns in the sEMG emerge depending on the task. This practical background leads us to believe that the mechanical advantages of using the dominant arm are not always applicable to all movements. However, the increasing in the sEMG activity of proximal muscles suggests a mechanism of stabilization of these joints, which would contribute to a minimization of trajectory deviations in relation to the target and so reducing the need of a over muscular recruitment beyond the necessary. Another important aspect concerns the consolidation of muscle patterns with training. This may be an important factor in discriminating the dominant arm, since a continuous use of a limb can conduct to modifications of the neural activity pattern related to the movement15.
In fact, the higher magnitude and variability of the ECR muscle activity observed during the performance of the manual task in the non-dominant arm can be an indicative of lower consolidation of motor synergies in the neural circuits. According to the concept of functional synergies proposed by Scholz e Schöner16, the human movement involves kinetic and kinematic patterns that favor its adaptation to the environment and conditions that could otherwise disrupt the movement itself. The muscular synergies are related to the myoelectric activity and kinematics patterns that favor the movement adaptation17. It is suggested that a higher magnitude and variability in the sEMG signal activity of non-dominant arm can be related to the presence of myoelectric activity/kinematics patterns that do not contribute to movement adaptation. This might be a result of a lower consistence of synergies of the non-dominant arm when compared to the dominant one.
Therefore, we conclude that the muscular recruitment of the non-dominant arm was significant higher both in magnitude and variability. Mainly, the higher RMS value shown by the ECR muscles during the performance of the required task with the non-dominant arm seems to express a higher muscular recruitment for this in hand movements, possibly increasing the energy expenditure of that task. Moreover, the RMS value seems to be a simple but effective method for myoelectric activity evaluation in paradigms like that employed in this study, which must be considered in attending athletes and patients.
Acknowledgments
T. Lemos is a recipient of a fellowship from CNPq (Brazil). The authors thank Keli Aragão for helping with the translation of the abstract in Spanish.
Correspondence:
M.A. Cavalcanti Garcia.
Núcleo de Estudos do Movimento Humano (NEMoH). Escola de Educação Física e Desportos, UFRJ.
Av. Carlos Chagas Filho, 540, Cidade Universitária, Ilha do Fundão.
Rio de Janeiro, Brasil, CEP: 21941-599.
E-mail: garcia@ufrj.br; lemos@biof.ufrj.br
History of the article:
Received April 18th 2010.
Accepted October 21th 2010.